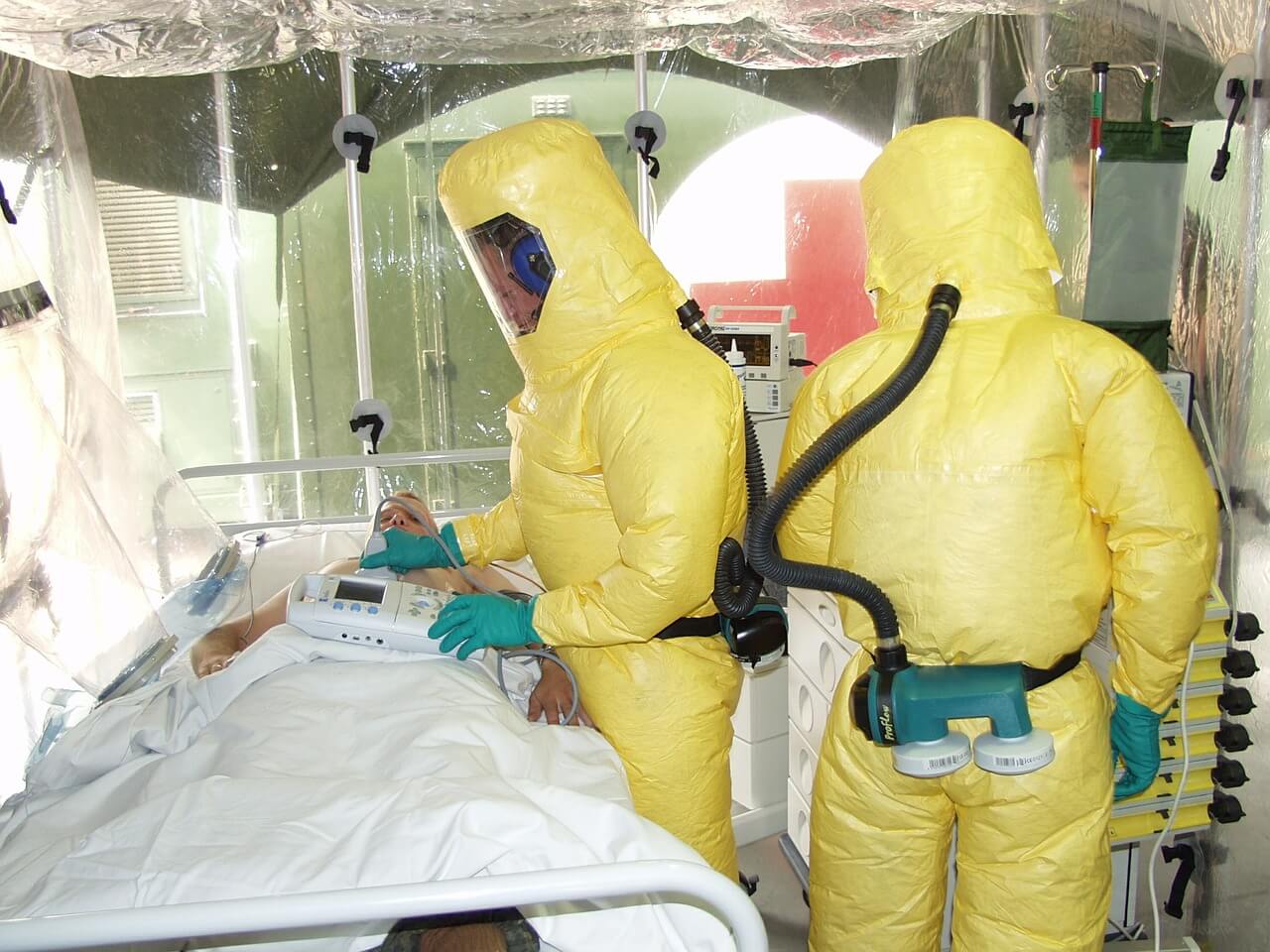
Ebola and Lassa fever targeted by new vaccine trial and improved surveillance
Scientists hope that a new approach to vaccine development, combined with improved surveillance of potential future threats of outbreak, could help to massively reduce the impact of deadly diseases such as Ebola, Marburg and Lassa fever.
„This has the potential to have an enormous positive impact on global public health“
Jonathan Heeney
Researchers from the University of Cambridge will shortly begin clinical trials of a new vaccine that builds on almost two decades of research to protect against diseases caused by RNA viruses. At the same time, they will begin studying the natural animal reservoirs of the viruses in an attempt to try and predict which strains are likely to cause future outbreaks, information that will be essential for creating effective vaccines.
Ebola, Lassa and Marburg viruses cause haemorrhagic fever, leading to severe disease, often with high mortality rates. Outbreaks can cause devastating local epidemics in the human population and to wildlife, including non-human primates. The recent Ebola epidemic in West Africa (2013–2016) killed over 11,000 people and devastated the infrastructure and economies of Liberia, Sierra Leone and Guinea.

A new approach to vaccine development
Professor Jonathan Heeney and colleagues at the Lab of Viral Zoonotics, University of Cambridge, have developed and successfully tested a trivalent vaccine in guinea pigs that protects against Ebola, Lassa and Marburg viruses. As a result, Professor Heeney has been awarded a further £2 million by Innovate UK and the Department of Health and Social Care to take the vaccine to clinical trials in humans.
The research takes a new approach pioneered by Professor Heeney and builds on Cambridge’s strengths in genomics, monoclonal antibody research and computational biology. It has led to the formation of DIOSynVax, a spin-out company of Cambridge Enterprise.
A virus’s genetic code is written into its RNA (just as ours is written into our DNA), which leads to the generation of proteins. When we are infected by a virus, our immune system responds to these proteins, known as ‘antigens’, producing antibodies that can identify and try to eliminate the invading pathogen.
The approach developed by Professor Heeney involves understanding how the immune system correctly identifies the virus from its proteins, and using this information to create ‘viruses’ that can generate an immune response. Using monoclonal antibodies – copies of antibodies taken from survivors of the target diseases – they can then test whether the body can effectively eliminate these fake viruses, leading to protection.
“We’ve taken fundamental science that stretches back almost two decades and developed a new approach to vaccine development,” says Professor Heeney. “This has the potential to dramatically reduce the time needed to produce new vaccines and change the way in which the industry makes them.”
With the new funding, the team hopes to scale up production while ensuring that the quality of the vaccine is maintained. They will then carry out toxicity tests in animals and human blood samples to test for potential adverse effects; if successful, they will then trial the vaccine in healthy human volunteers.
The funding is part of a £5m commitment from the Department of Health and Social Care to fund five projects to develop new vaccines with a ‘One Health’ focus, considering how the environment, the health of animals and the health of humans interact. This sits within the government’s £120m UK aid commitment to develop vaccines to help tackle diseases with epidemic potential.
Predicting the next outbreak
In recent Ebola outbreaks, the approach used successfully by the World Health Organization is known as ‘ring vaccination’, focused on vaccinating and monitoring a ring of people around each infected individual. However, this approach can only be used in response to an outbreak. In order for a vaccine to be used proactively – to prevent an outbreak in the first place – it is necessary to predict which strain or strains of a virus are most likely to cause future epidemics.
“A disproportionally high number of emerging and re-emerging diseases – from Ebola and Lassa through to rabies and influenza – are caused by RNA viruses carried naturally by animals,” says Professor Heeney. “We know very little about the viral diversity within these reservoir species and what enables them to spread to humans – and hence where the likely future threats lie.”
Viral genomes are notoriously variable due to the high mutation rates that occur during replication. These accumulate over time and result in evolution of the viruses as they circulate in their natural animal reservoir populations. If some viral variants arise and are able to adapt to use human cell receptors and are then able to escape immune defences, they may become highly infectious and cause large disease outbreaks.
“Vaccines are only as good as the antigen immune targets of the virus that they are designed for,” adds Professor Heeney. “If the antigen changes, the vaccine will no longer be effective. In most cases, current vaccine candidates against RNA viruses are from past human outbreaks with little or no information of future risks from viral variants carried in animal reservoirs, especially those with the potential for animal-to-human transmission.”
Professor Heeney has also received £1.4 million from the Biotechnology and Biological Sciences Research Council (BBSRC) to lead a project that aims to predict where future outbreaks may arise from and the likely strains, and to then use this knowledge to inform vaccine design. This One Health project enlists veterinarians, clinicians, ecologists and medical and public health workers in West Africa to understand how people catch Lassa fever from rat populations. Their work will include trapping rat species that carry these viruses and placing GPS tags to monitor their movements, as well as obtaining molecular, genomic and antibody data from the animals and viral sequences from infected rats.
Professor Melanie Welham, Executive Chair of BBSRC, says: “This important research from the team at the University of Cambridge is about providing effective treatments for some potentially deadly diseases spread by rats and bats: Lassa and Ebola respectively. Novel strategies to combat dangerous infections like these are essential and often underpin the development of much-needed next generation vaccines.
“Professor Heeney and team have already made a significant difference in this area, researching cross species transmissions of these viruses, with a view to developing vaccines for Ebola and Lassa that would be effective against multiple strains.”
In addition, the team is collaborating with Professor James Wood, Head of the Department of Veterinary Medicine at Cambridge, who is conducting a complementary study funded by the Global Challenges Research Fund to sample bat colonies in Ghana, believed to be a natural reservoir for the Ebola virus.
“Equipped with this information, we should be able to design better vaccine antigens for more effective and broadly-protective vaccines,” says Professor Heeney. “Combined with our accelerated vaccine development platform, this has the potential to have an enormous positive impact on global public health.”

The text in this work is licensed under a Creative Commons Attribution 4.0 International License.